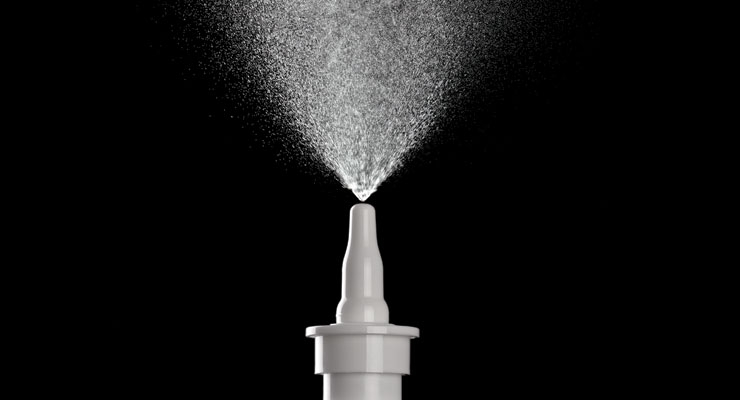
Nasal spray devices are used to deliver both locally and systemically acting pharmaceutical products,1 and are readily available in the marketplace in unit dose and metered multi-dose formats. Unit dose devices are used to treat conditions such as migraine pain (Imitrex and Zomig), cancer pain (Instanyl), vitamin B12 deficiency (Nascobal), and heroin overdose (Narcan); multi-dose devices are for conditions such as allergy (Flonase, Nasacort), sinus congestion (Mucinex Sinus, Similisan Nasal Allergy Relief), cold relief (Zicam), migraine pain (Migranal), pain management (Sprix) and nocturia (Noctiva).
Although some interest has been shown relating to bi-dose nasal devices, they are currently less common.
One of the benefits of nasal delivery for systemic drugs is that they can bypass the blood-brain barrier and enter the brain directly via the olfactory and trigeminal nerve pathways.2-4 Additionally, the nasal cavity has a thin, well vascularised mucosa.5 Drug molecules can transfer across this layer directly to the systemic blood circulation6,7 thereby avoiding first-pass hepatic and/or intestinal metabolism.
Spray Characterization Tests
For all spray characterization tests, the spray device is actuated using controlled parameters—force, speed, acceleration, stroke length.
Spray Pattern. The shape of the plume from a bird’s eye view when looking directly towards the actuator orifice as the product is emitted from the device. Testing is performed at fixed distances from the actuator tip, and the spray pattern is characterized using the following parameters:
- Dmax—the length of the longest line that passes through the weighted center of mass drawn within the perimeter of the spray;
- Dmin—the length of the shortest line that passes through the weighted center of mass drawn within the perimeter of the spray;
- Ovality ratio—the ratio of Dmax/Dmin;
- Ellipticity—the ratio of the major and minor axes of the ellipse; and
- Area—the cross-sectional area defined by the spray.
- Plume Geometry—the shape of the plume when looking at the spray from the side as the product is emitted from the device. The plume geometry is characterized using the following parameters:
- Plume Angle—the angle created at the base of the plume as the product is emitted from the actuator orifice; and
- Plume Width—the width of the plume at a specified distance from the actuator tip.
The pump design, size and shape of the actuator orifice, size of metering chamber (for multi-dose devices), and formulation characteristics (e.g., viscosity10) can affect the spray pattern and plume geometry.
Single Actuation Content/Delivered Dose Uniformity.11 A measure of the amount of active ingredient contained within individual sprays across the lifetime of a single device for multi-dose sprays, and between devices produced throughout the course of a packaging run—e.g., from the beginning, middle, and end of the filling process. The emitted spray is captured, and the content of active ingredient, or other functional ingredient, is assayed using the normal product assay method (e.g., HPLC).
This testing is performed to ensure that the dosage per actuation complies with the stated label claim, does not change from one actuation to the next, and does not vary from one device to the next.
Droplet Size Distribution.11 The droplet size within the spray plume in terms of cumulative volume distributions:
- Dv10, Dv50, Dv90—the volume median diameter (or Dv50) value indicates that half of the spray volume is contained in droplets that are smaller than this value, and half is contained in droplets that are larger than this value. Similarly, the Dv10 and Dv90 values indicate that 10% and 90%, respectively, of the spray volume is contained in droplets that are smaller than these values;
- Span—the span quantifies the spread of the droplet size distribution and is calculated by the following equation: (Dv90 − Dv10)/Dv50; and
- %<10 μm—the percentage of droplets that are less than 10 μm in diameter.
Droplet size is related to the ease of atomization of the formulation. A decrease in viscosity or surface tension should help the formulation to atomize and give rise to smaller droplets10 and, consequently, a larger spray pattern surface area.
For nasal sprays, droplets that are excessively large (Dv90 > 300 μm) will have a tendency to either drip out of the nose,12 or hit the back of the throat and be swallowed. The value for %<10 μm provides a risk estimate of small droplets that may be deposited into the lung. Neither of these situations is the intended route of administration and will, therefore, have an impact on the effectiveness of the delivered dose.
Dose Weight. Dose weight, or pump delivery, testing is performed to assess pump-to-pump reproducibility both within a given product batch, and between different batches of product. The amount of product dispensed by the device will have a direct impact on the efficacy, and safety, in the case of over delivery, of the product. The acceptance criteria in USP<698> (Delivered Volume) may be used as a basis for this testing.
Priming and Repriming (Effect of Resting Time). Typically, multi-dose metered nasal spray devices have a dip tube going from the actuator into the product. This is to enable liquid product to be delivered when the device is used in an upright orientation. The device will need to be primed by actuating several times to fill the dip tube before the full dose is emitted. The number of actuations needed to prime the device is obtained by measuring the emitted dose weight until the full (label claim) dose is achieved.
During normal use, these devices will slowly “lose” their prime. That is, the product in the dip tube will slowly run back out into the product container. The time period after which the device needs additional priming actuations to deliver the full dose, and the number of actuations needed to reprime, should be determined.
Effect of Dosing Orientation. To cover the case where a user might not actuate the nasal spray in an upright orientation, nasal characterization testing should be performed at several different angles from upright/vertical. This is particularly important for multi-dose devices where air might get sucked into the dip tube, thereby reducing the delivered dose.
Number of Doses and Tail-Off Characteristics. The number of doses contained in a multi-dose device will depend on the actuation volume, the priming needs, and the container fill weight. This testing is performed by recording the weight of each actuation until the container is exhausted.
After the stated number of “useful” doses has been expelled some product will remain in the container. The profile of the sprays—spray content uniformity, droplet size distribution—should be determined immediately before and then after the labeled number of doses have been expelled until the container is exhausted.
Other Tests
Particle Size Distribution (Suspensions). For suspension nasal sprays, the size distribution of the drug substance particles in the formulation should be determined. Furthermore, if the formulation contains a suspending agent (e.g., Avicel from FMC BioPolymer) the method used must be able to distinguish between the drug particle and the suspending agent. Smaller drug substance particles have a relatively larger surface area. A larger surface area will affect the rate of dissolution and absorption of the drug, thereby influencing the pharmacokinetics.
Particulate Matter. The presence of particulate matter, which can originate during manufacturing from formulation components, container and closure components, and the manufacturing environment, needs to be assessed. Typically, the cumulative number of particles ≥ 10 µm and ≥ 25 µm, and in some instances ≥ 50 µm, are determined. USP<788> (Particulate Matter in Injections) and USP<789> (Particulate Matter in Ophthalmic Solutions) outline applicable testing procedures.
Device Robustness. The performance characteristics of the device should be studied after different handling situations. Baseline testing should be performed prior to commencing the studies. Product in its shipping or shelf container should be shipped, pre-conditioned at different temperatures/humidities, dropped,13 and vibrated14 before being re-tested to determine any change in performance resulting from possible in-market events.
General Tests. In addition to the above, the following list of general formulation tests may also be required: description; identification; assay; impurities and degradation products; preservatives and stabilizing excipients assay; microbial limits; net content; weight loss (stability); leachables (stability); pH; osmolality; viscosity; temperature cycling; and photostability.
Examples of Equipment
Spray Pattern/Plume Geometry. Measured using a non-impaction laser sheet-based instrument. A high speed camera is used to capture the interference pattern of the spray with the laser sheet. Instrument suppliers include Proveris Scientific Corp. (Marlboro, MA), InnovaSystems Inc. (Moorestown, NJ) and Oxford Lasers Inc. (Shirley, MA).
Droplet Size Distribution. Measured using a laser diffraction system. As the nasal spray droplets pass through the laser beam, the smaller droplets diffract the laser light at larger angles than the larger droplets. The diffracted light is captured on a series of concentric photodetectors, and then converted into a measurement of the droplet size. Instrument suppliers include Malvern Panalytical Inc. (Westborough, MA) and Sympatec Inc. (Pennington, NJ).
Particle Size Distribution and Particulate Matter. USP<788> (Particulate Matter in Injections) lists methods for determining particle count by light obscuration (Method 1) and by microscopy (Method 2).
The light obscuration method uses a particle sizer to determine the size and number of particles in a solution by measuring the amount of light a particle blocks or scatters when it passes through the detection window area of the instrument. Examples of instruments include the HIAC range (Beckman Coulter Life Sciences, Indianapolis, IN), the Mastersizer range (Malvern Panalytical Inc., Westborough, MA), and particle sizers from Shimadzu Scientific Instruments (Somerset, NJ) and Horiba Scientific (Ederson, NJ).
Microscopic evaluation can provide information on the size of particles, the extent of agglomerates, and particle morphology. Microscopy can also be used to determine different types of particles in a sample, for example, drug particles and suspending agents. The drawback with microscopy is that it is a manual technique and, therefore, time consuming.
Morphological imaging instruments are available which enable the automated measurement of particle size, shape and chemical identity. These instruments use a combination of image analysis and morphologically-directed Raman spectroscopy. Examples of this type of instrument are the Morphologi range of instruments from Malvern Panalytical Inc. (Westborough, MA).
Renaissance Lakewood, LLC has experience in developing, qualifying and validating methods to characterize the spray characteristics of nasal sprays in addition to transferring and validating methods which have been developed at third party facilities. Visit www.renpharm.com to learn more.
References
- J.D. Ehrick, S.A. Shah, C. Shaw, V.S. Kulkarni, I. Coowanitwong, S. De and J.D. Suman. Chapter 5, “Considerations for the Development of Nasal Dosage Forms” in “Sterile Product Development: Formulation, Process, Quality and Regulatory Considerations”. AAPS Advances in the Pharmaceutical Sciences Series, Volume 6, 2013, pp 99-144. Springer NY
- Intranasal Drug Delivery Bypasses the Blood–Brain Barrier. Neurology Reviews, 2016 April; 24(4):1, 40-41
- W. Ying. The nose may help the brain: intranasal drug delivery for treating neurological diseases. Future Neurol 2008; 3(1): 1-4
- S.V. Dhuria, L.R. Hanson and W.H. Frey. Intranasal delivery to the central nervous system: Mechanisms and experimental considerations. J Pharm Sci 2010; 99(4): 1654-1673
- D.F. Proctor and I. Andersen. The nose. Upper airway physiology and the atmospheric environment, Elsevier Biomedical Press, Amsterdam, 1982
- B.L. Laube. Devices for aerosol delivery to treat sinusitis. J Aerosol Med 2007; 20: S5-S17
- S.P. Newman, G.R. Pitcairn and R.N. Dalby. Drug delivery to the nasal cavity: In vitro and in vivo assessment. Crit Rev Ther Drug Carrier Syst 2004; 21: 21-66
- FDA Guidance for Industry: Nasal Spray and Inhalation Solution, Suspension, and Spray Drug Products – Chemistry, Manufacturing, and Controls Documentation. July 2002
- V.S. Kulkarni and C. Shaw. Essential Chemistry for Formulators of Semisolid and Liquid Dosages. Elsevier (Academic Press) USA, 2015: 211-216
- V.S. Kulkarni, C. Shaw, M. Smith and J. Brunotte. Characterization of plumes of nasal spray formulations containing mucoadhesive agents sprayed from different types of device. Poster presentation, AAPS annual meeting, San Antonio, Texas. November 2013
- USP<601> Inhalation and nasal drug products: Aerosols, sprays, and powders – performance quality tests
- V. Kulkarni and C. Shaw. Formulation and characterization of nasal sprays. Inhalation June 2012: 10-5
- ASTM D5276-98. Standard Test Method for Drop Test of Loaded Containers by Free Fall
- ASTM D 999-08. Standard Test Methods for Vibration Testing of Shipping Containers
Article from: https://www.contractpharma.com/issues/2019-01-01/view_features/nasal-drug-delivery

 Nose, Throat, Ear
Nose, Throat, Ear Ophthalmic
Ophthalmic Inhalation
Inhalation Gynecology
Gynecology Vaccine
Vaccine Bottles+IVD
Bottles+IVD