We are thrilled to share the exciting news that our valued partner, Dr. Ferrer Biopharma, has been honored with the prestigious Patient-Centric Design Award at the 2024 Pharmapack Awards. As the manufacturer partner at BONA, we take immense pride in contributing to this achievement and shaping the future of pharmaceutical packaging and drug delivery. About

Author: bona01
BONA presents disposable intranasal atomization device
BONA Pharma has developed an atomizing device for drug administration intended to convert liquid formulations into atomized particles to be sprayed on the surface of human body tissues or organs, meaning it provides full contact and maximizes its application capabilities. Moreover, the carefully designed self-destructive structure ensures the product can only be used once to
BONA is well prepared to develop nasal pump for both originator and generics
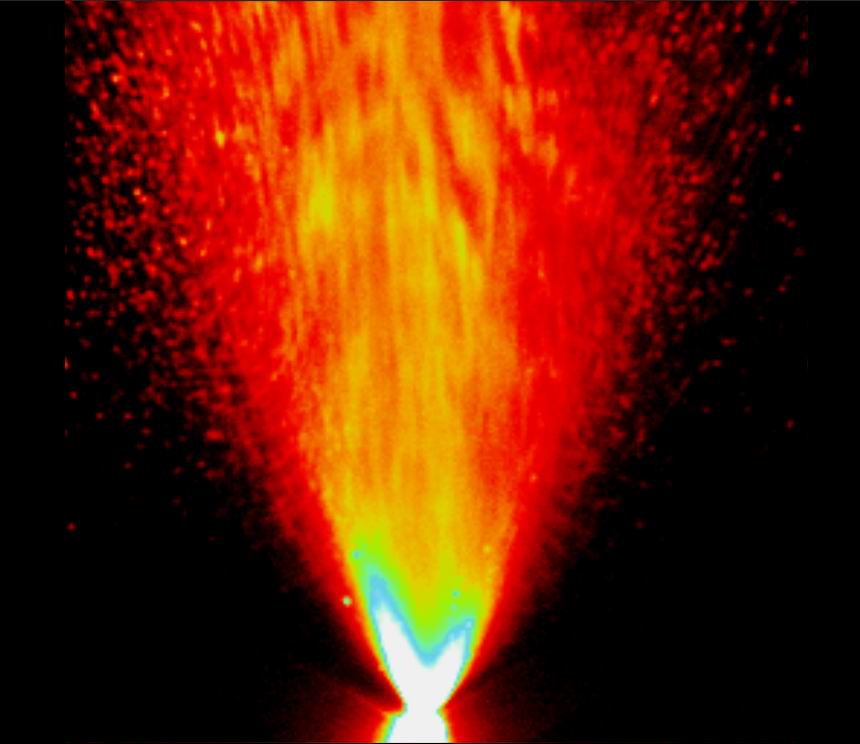
After the new automated factory was put into operation in 2019, BONA grows increasingly rapid. In the past years, we had not only upgraded the production lines, but also improved our Quality Management system. Moreover, huge invest in R&D makes BONA products perfect for customers. With the introduction of spray performance analytical devices, our engineers
Renewal-audition for ISO 15378
After 5 days of renewal-audition for ISO 15378 by SGS, we finally get the approval result. This audition started from June 6 and ended on June 10. This time, the SGS auditors gave full affirmation to thecontinuous improvement of BONA quality management system in recent years. We are very happy to share our latest results

 Nose, Throat, Ear
Nose, Throat, Ear Ophthalmic
Ophthalmic Inhalation
Inhalation Gynecology
Gynecology Vaccine
Vaccine Bottles+IVD
Bottles+IVD